
Solar Panels
The solar panel industry is one of the largest and most successful renewable energy industries in the world. This success is due to the effectiveness of solar panels both as a developing technology and as a dynamic means to create electricity. Because the amount of sunlight that hits the earth in one minute contains more energy than is consumed by the global population in one year, 1 photovoltaics (PV) is seen as one of the major long term energy technologies for the future. The International Energy Agency (IEA) has predicted that by 2030 photovoltaics will generate 5% of global electricity production, and by 2050 this will become 11%. 2 (pg. 7)
Solar panels are effective because they:
- Are easy to install and maintain
- There are no moving parts
- Have a long lifetime (20 – 25 years)
- Can be used in remote off-grid locations
- Are close to grid parity (when an alternative energy technology is equal to or cheaper than electricity that comes from the grid)
- Efficiencies are rising as production costs are falling
- Recover their costs over time
Currently there are three main types of solar cells being produced. These are all made with silicon, and each have advantages and disadvantages as follows:
- Monocrystalline cell
- Made from a single silicon crystal
- More expensive
- More efficient
- Rigid structure limits the form to a flat surface
- Polycrystalline (or Multicrystalline) cell
- Made from multiple silicon crystals
- Less expensive
- Less efficient
- Rigid structure limits the form to a flat surface
- Ribbon silicon cell
- Made from molten silicon that results in a multicrystalline structure
- Less expensive
- Less efficient
- Amorphous structure allows for multiple surface types
Solar Cell Research and Development
Due to the enormous potential that exists for PV as an efficient and a profitable technology, there is large scale investment in its research and development. This includes advancements in the existing silicon methods as well as with new materials. The following is a list of developing PV technologies (from Wikipedia article):
- Silicon processing
- Thin-film processing
- Metamorphic multijunction solar cell
- Polymer processing
- Nanoparticle processing
- Transparent conductors
- Silicon wafer-based solar cells
- Infrared solar cells
- UV solar cells
- 3D solar cells
- Metamaterials
- Photovoltaic thermal hybrid
Photovoltaic operation is based on photons (see Atoms, Electrons and Photons article this website), which are created when an electron in a higher than normal orbital moves down to its normal (lower) orbital in an atom. Once emitted from an atom photons travel as light through space and when they hit matter several things can happen depending on the amount of photon energy and the type of matter hit.
- The photons can be reflected or scattered off the object
- The photons can be absorbed by the object
- The photons can be refracted through the object
- The photons can pass through the object with no effect
See video below.
For our purposes we will only focus on the effect of absorption, which then creates the Photoelectric Effect or more specifically the Photovoltaic Effect. See below video of the Photoelectric Effect:
The Photoelectric Effect, proposed by Einstein, is the condition whereby photons hitting matter cause the ejection of electrons from the atoms of the particular matter. This process is reverse to the process of creating photons, i.e., instead of electrons moving to lower less energized orbits, they are moved up to higher more energized orbits, and can ultimately be ejected altogether.
Whether electrons are ejected or not from the matter hit, depends on the matter’s band gap energy, which dictates how much photon energy is required to eject an electron from an atom of that particular matter. Certain materials (matter) eject electrons more easily than others.
In the case of solar panels, this effect is used to generate electricity as follows:
Currently there are many materials and composite systems being developed to turn photon energy into electricity. To grasp the basics of this process the description below relates to how this is done for the most common solar cells, which use silicon.
The silicon atom has three levels of electrons. The two inner levels are full, but the outer most level is only half full, containing four electrons, but having space for eight. It gets the missing 4 electrons by sharing one from each of 4 other silicon atoms, and this sharing creates a bond of crystalline structure. As such, photons hitting silicon do not have much useful effect because freeing up electrons is not easy. However, by mixing impurities (called doping) into the silicon structure it is possible to better control the photoelectric effect to create electricity.
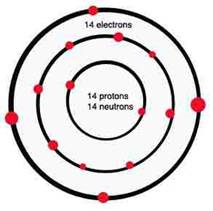
Silicon atom Silicon molecule
This is done by creating two parts, which together create a diode. The first part is a mix of silicon and phosphorous that creates a negative side called the N-Type (N for negative), and the second is a mix of silicon and boron that creates a positive side called the P-Type (P for positive).
The phosphorus atom is structured like the silicon atom except that it contains five electrons in its outer most level instead of 4. When mixed with silicon the result creates an extra electron (4 + 5 = 9 – 8 = 1), which is constantly searching for a place to fit in.
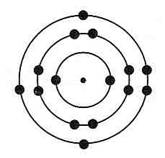 Phosphorus atom
Phosphorus atom
The boron atom has two levels of electrons, with the outer most level containing three electrons. When boron is mixed with silicon the result creates a proton hole (4 + 3 = 7 – 8 = -1) for an extra electron to fit in.
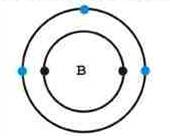 Boron atom
Boron atom
When these two positive and negative parts are placed close together a flow of free electrons from the N-Type side seeking free proton holes from the P-Type side is started.
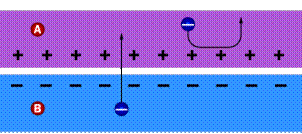
Free N-Type electrons moving to the P-Type side to find proton holes happens in one direction, where, free P-Type electrons cannot return to the N-Type side because there are fewer proton holes to receive free electrons on that side. This creates the diode with unidirectional electron flow and ultimately an electrical current.
When photons of light hit this system they intensify the process of freeing electrons to the point that there are enough free electrons moving in one direction to create a useable electrical current.
Once the current is created it can be directed by wires to a battery, which receives the incoming electrical charge and stores it. This system creates direct current (DC) electricity.
References
- http://www.altenergy.org/renewables/solar.html Alternative Energy
- http://iea.org/papers/2010/pv_roadmap.pdf International Energy Agency, pg. 7
Links
http://en.wikipedia.org/wiki/Photovoltaics
http://en.wikipedia.org/wiki/Solar_cells
http://en.wikipedia.org/wiki/Solar_panel
http://en.wikipedia.org/wiki/Photovoltaic_effect
http://science.howstuffworks.com/environmental/energy/solar-cell.htm





