
Atoms, Electrons and Photons
Important terms
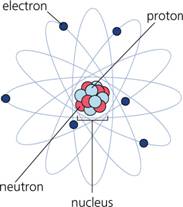
- Proton
- A positively charged atomic element
- Neutron
- A neutrally charged atomic element
- Nucleus
- The center of an atom, composed of the proton(s) and the neutron(s)
- Electron
- A negatively charged atomic element that orbits the nucleus
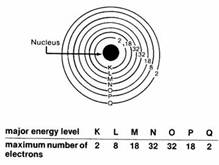
- Electron shell
- The orbital level(s) that an electron can occupy
- Electromagnetic force
- One of the four fundamental forces of nature that binds electrons to the nucleus
- Photon
- The basic unit of light and the force carrier for the electromagnetic force
- Ion
- An atom that has either gained or lost an electron, causing the atom to become either negatively or positively charged, respectively
The atom is composed of a nucleus at its center, which contains positively charged protons and neutrally charged neutrons. Moving about the nucleus are electrons, which are negatively charged and are bound to the nucleus by the electromagnetic force.
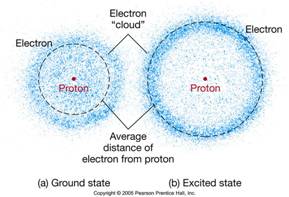
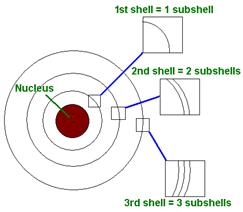
Electrons are often represented as orbiting the nucleus, but in reality their movement is more complex and they are considered to move about in an “electron cloud”, which exists around the nucleus. Within the electron cloud there are different shells (energized levels) that an electron can occupy. A stable atom has an equal number of protons, neutrons and electrons. As such it has a neutral charge.
There has been debate about charge assignment, see the following article about conventional flow notation versus electron flow notation:
http://www.allaboutcircuits.com/vol_1/chpt_1/7.html
Electron configuration in most atoms involves electron pairs, i.e., two electrons occupying the same shell level, as opposed to just one electron, a state which also exists. In an electron pair each electron has an opposite spin to the other, which causes them to cancel out each other’s magnetic influence and creates magnetic neutrality for the atom.
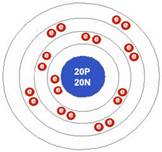 Calcium atom
Calcium atom
“The outermost orbital shell of an atom is called its valence shell, [and the electrons there are called valence electrons]. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don’t participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table.” 1
Electrons in the outermost valency shell are the furthest from the nucleus and therefore have the weakest attraction to it. In certain conditions the outermost valency electron is so loosely bonded to the atom, e.g., the copper atom, that it is free to randomly wander from one atom to another. This creates free electrons. When the random movement of free electrons is controlled by an external influence, e.g., a magnet, the free electrons can be directed to form an electrical current.
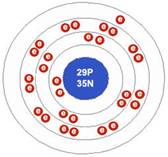 Copper atom
Copper atom
The electron is responsible for chemical bonding where electrons are shared between atoms to create molecules. There are two types of bonding, covalent and ionized.
Covalent bonding involves atoms sharing valency electrons, usually in atoms which have only one electron in their valency shell, and so seek stability by adopting a second electron, in this case by sharing it with another atom that also seeks this stability.
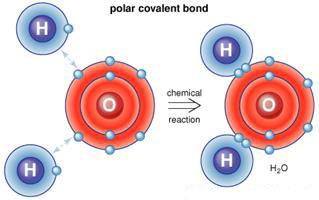 Water molecule
Water molecule
Ionic bonding involves one atom losing an electron to another atom. The first becomes a positively charged ion and the second becomes a negatively charged ion. This charge difference creates an attraction between the two ions and they join to form a bond.
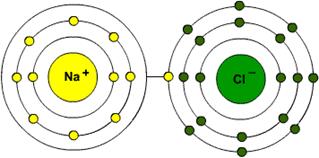 Sodium chloride molecule (salt)
Sodium chloride molecule (salt)
“An electron has a natural orbit that it occupies, but if you energize an atom you can move its electrons to higher orbitals [shells]. A photon of light is produced whenever an electron in a higher-than-normal orbit falls back to its normal orbit. During the fall from high-energy to normal-energy, the electron emits a photon — a packet of energy — with very specific characteristics. The photon has a frequency, or color, that exactly matches the distance the electron falls.” 1
The reverse process, i.e., when an electron moves to a higher orbital (shell), happens when a photon impacts with an atom, i.e., the atom absorbs external energy.
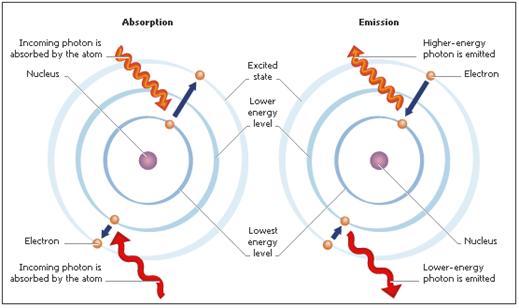
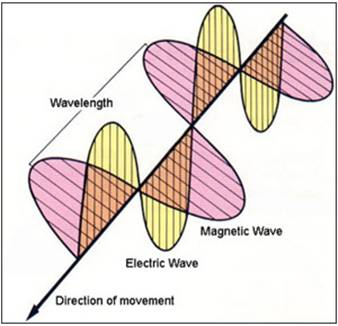
Currently light is known as being of wave forms called fields and particle forms called photons.
Field (wave) properties include:
- 1 electric field (wave)
- 1 magnetic field (wave)
- Each field (wave) is perpendicular to the other and both are perpendicular to the direction of movement (see below image)
Photon properties include:
- No mass
- No electric charge
- No magnetic charge
- A speed
- Angular momentum (like a gyroscope) with spin axis parallel or anti-parallel to its direction of movement
Light travels as photon particles that also create a field-wave of electromagnetic radiation, i.e., it is photo-electromagnetic energy.
Photons carry energy (photon energy) relative to their frequency (wavelength) of light, where, for example, red light, which has a long wavelength, has low photon energy, and ultra-violet light, which has a much shorter wavelength, has higher photon energy.
When photons hit matter, several things can happen depending on the amount of photon energy and the type of matter hit. See video below.
- The photons can be reflected or scattered off the object.
- The photons can be absorbed by the object.
- The photons can be refracted through the object.
- The photons can pass through the object with no effect.
This behavior is important for understanding solar panel technology (see Solar Panels article).
Links
http://en.wikipedia.org/wiki/Atom
http://en.wikipedia.org/wiki/Electron





